Blog | Reading Time 6 minutes
TITAN technology: Ensuring quality and viability of live yeast
 Probiotic yeast needs protection
Probiotic yeast needs protection
Many experts insist that probiotics must be alive and viable to exert an effect on the microflora within the digestive system. Indeed, several national authorities will only authorize a claim for performance (such as improved milk production or feed efficiency) when the probiotic microorganism is viable. For example, the European Commission considers microorganisms as probiotic feed additives only in their live form.
 Today, the increased demand for pelleted feed, coupled with increasingly stringent feed mill processes, could limit the inclusion of probiotic yeast in this form of feed. This is why Lallemand continues to invest in process development to ensure that TITAN, its unique patented yeast protection technology, remains at the top of the game when it comes to withstanding stringent pelleting conditions.
Today, the increased demand for pelleted feed, coupled with increasingly stringent feed mill processes, could limit the inclusion of probiotic yeast in this form of feed. This is why Lallemand continues to invest in process development to ensure that TITAN, its unique patented yeast protection technology, remains at the top of the game when it comes to withstanding stringent pelleting conditions.
The stress of feed pelleting
When selecting a probiotic yeast, it is crucial to ensure the live yeast will: survive feed or premix processing; tolerate the combination of other ingredients; and endure storage prior to reaching the animal. Feed manufacturing processes are constantly evolving to answer market needs and safety challenges. Feed pelleting processes represent many stress factors for live yeast, such as temperature, pressure and/or moisture. For example, yeast stability depends on the die temperature during the pelleting process, but also on the die compression, conditioner temperature and process duration.
TITAN offers optimal stability
TITAN is a live yeast protection technology adapted for pelleted feed applications that has been developed by Lallemand Animal Nutrition.
TITAN includes a unique and patented yeast microencapsulation technology (Read Jean-François Hupé’s interview here to know more).
In addition to this unique coating, the production process of TITAN includes specific adaptation of the live yeast fermentation and drying conditions, as well as downstream processing steps up to packaging to ensure optimal resistance and viability of the live yeast in customer applications.
As a result, TITAN offers a consistent and optimal stability to Lallemand Animal Nutrition live yeast probiotics during industrial feed processing steps and through feed storage.
Analysis were conducted by an independent institute, the International Research Association of Feed Technology (IFF) in Germany, in various pelleting conditions to compare the stability and the resistance of a TITAN live yeast form to other commercial yeast sources under different pelleting conditions. This study showed that only TITAN yeast remains stable throughout the various pelleting processes.
A specific industrial process
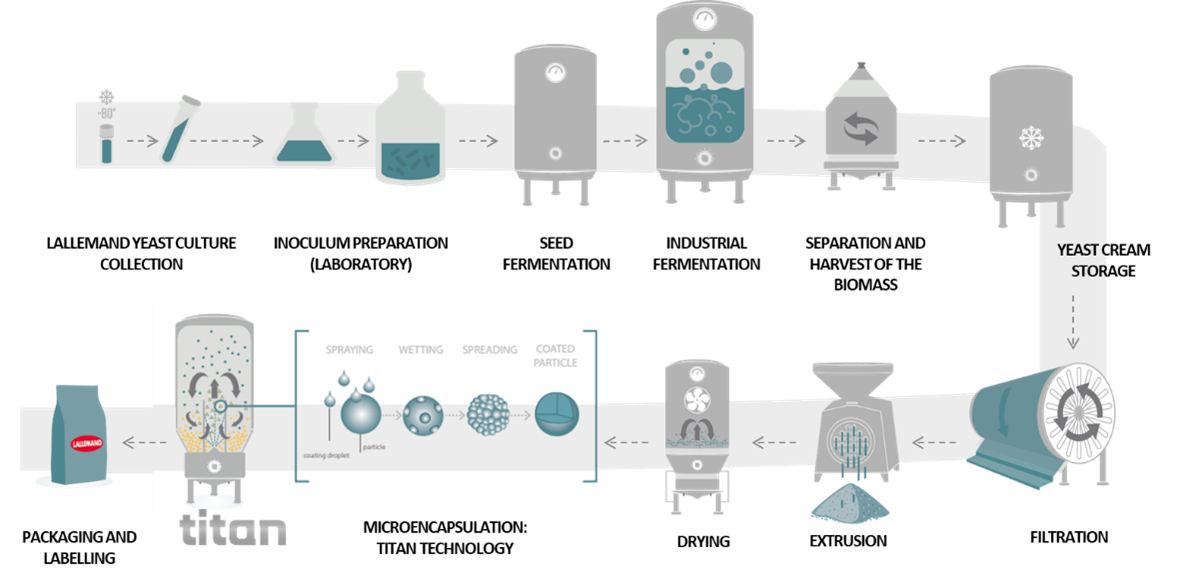
Producing quality yeast requires skills and expertise, the Lallemand group has mastered the art of fermentation during its 100+ years of experience in yeast and bacteria production across a broad range of markets. As a primary producer of microorganisms, the company has a full control over the production process from the lab to packaging. Moreover, strict quality control is implemented at every stage from the cell bank that is used to start production to the logistics platform. This ensures the quality, safety and consistency of our TITAN protected live yeast for all customers and end-users.
The main challenges of yeast production are to ensure proper yields and outputs (live biomass) and optimal performance (metabolic activity) of the end-product for users, while preventing the presence of contaminating microbes. These objectives can be accomplished thanks to meticulously controlled conditions and tight quality control along the process. In particular, all physico-chemical parameters such as medium composition, temperature, pH, nutrients concentration and agitation are strictly controlled during the whole process. These parameters are determinant for the product’s final activity and efficacy, and each yeast strain has its own optimal conditions. Hence, for each strain these conditions have been carefully determined at lab and pilot scales before industrialization.
The parameters are thoroughly controlled from seed to final formulation to ensure consistency of the final product.
Each and every step of yeast production process can impact its viability. Thus, when the process optimization team at Lallemand worked on developing TITAN, they scrutinized the whole process. They adapted the fermentation and drying processes and combined this with an extra step: a protective microencapsulation.
When producing TITAN yeast, the fermentation and drying conditions are specific. Following fermentation, the conventional active dry yeast (ADY) process is typically extruded into a vermicelli particle form. This form of ADY is suitable for less stringent applications such as mash feed.
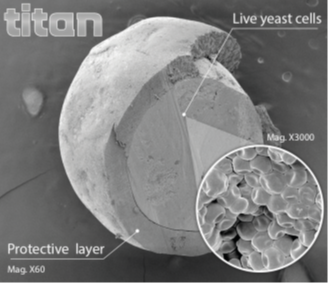
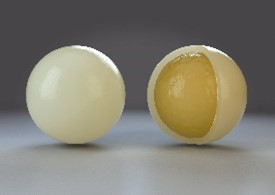
For TITAN production, the yeast particles are submitted to a particular drying process to form beadlets. The dried beadlets are then coated in a batch fluid bed with a specifically formulated lipid-based solution.
This coating step, or microencapsulation, specific to ADY has been patented (EP 2099898B1) in 2007. TITAN is a unique yeast microencapsulation technology in animal feed additives.
Ensuring quality and safety all the way
Throughout the whole production process, about 20 quality tests are performed, ensuring purity, viability and identity of the yeast strain in the final product, as well as batch-to-batch consistency. A certificate of analysis is released for every batch of live yeast produced by Lallemand.
Moreover, quality control does not stop at the plant gate. Lallemand quality team also conducts regular customer products analysis to ensure the live yeast they purchase remains viable and active in its final application, such as feed, premixes, pellets and more.
Lallemand Quality Assurance System
All Lallemand Animal Nutrition production sites and suppliers operate under the FAMI-QS or equivalent certification system, which includes:
- GMP (good manufacturing practices)
- HACCP (hazard analysis and critical control points)
- FFFD (feed fraud vulnerability and feed defense)
This certification is the object of annual audits and renewed every three years.
It ensures compliance with EU Feed Hygiene Regulation (183/2005/EC), US FDA FSMA and most country requirements in terms of Quality and Safety Certification. These rigorous quality assurance procedures safeguard that final products are safe, of the highest quality, and compliant to customers’ requirements.
Finally, in order to ensure continuous improvement of its products’ safety, quality and efficacy, Lallemand group operates a centralized complaint system. Feedback from customers is vital in this process and so all complaint information is communicated via this system to the facilities involved as well as to senior management from the business unit and the corporate Lallemand Group, from submission to complaint resolution.
Lallemand technical services
Lallemand Animal Nutrition has an international process development platform as part of its R&D department, dedicated to continuous process optimization as well as the applications of TITAN.
Under a large variety of feed processing conditions, the feed industry can benefit from on-site support to assist in feed process monitoring and sampling. The company’s feed industry partners have access to dedicated services such as sample analysis and compatibility studies. All this helps ensure optimal viability and efficacy of Lallemand’s probiotic yeast to end-users.
You want to know more about our QC/QA system? Read the interview with our Quality Manager here!
Published Apr 25, 2023 | Updated May 29, 2023